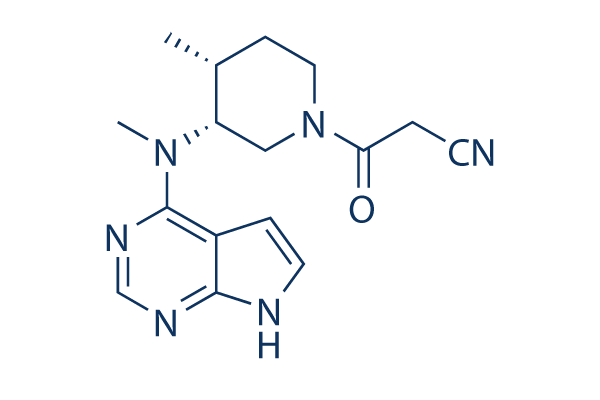
Xeljanz XR (tofacitinib citrate) Modified Release Tablets For the Treatment of Rheumatoid Arthritis - Clinical Trials Arena

Pfizer Announces FDA Approval of XELJANZ® XR (tofacitinib citrate) Extended-Release Tablets, the First and Only Once-Daily Oral JAK Inhibitor Treatment for Rheumatoid Arthritis | Business Wire
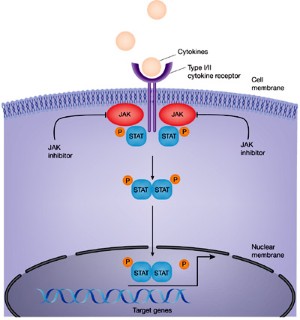
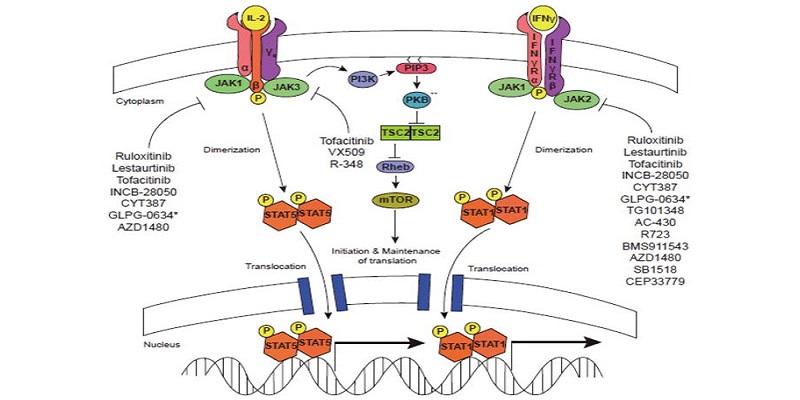
JAK inhibition as a therapeutic strategy for immune and inflammatory diseases | Nature Reviews Drug Discovery
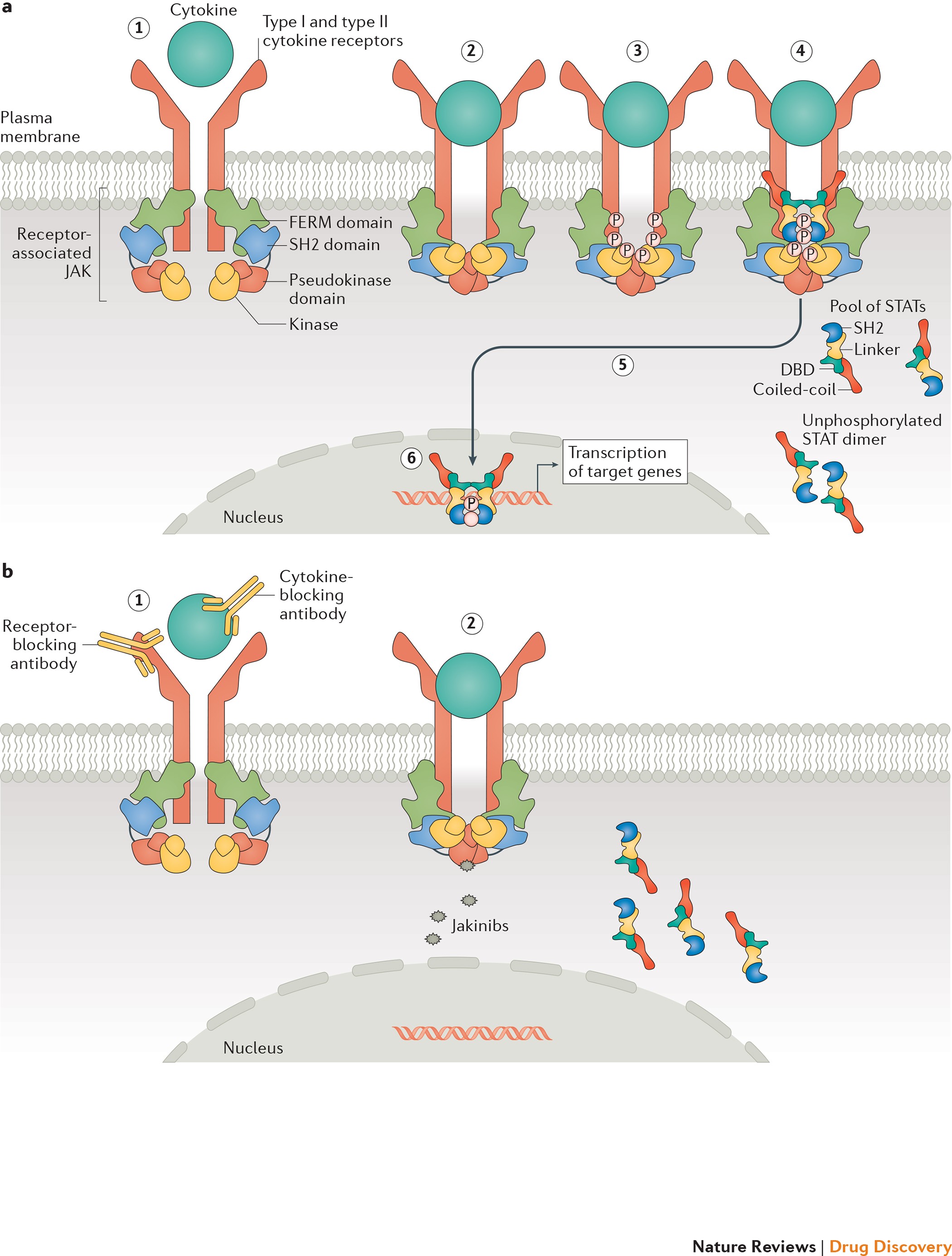
FDA Crisis for Pfizer: The Impact of an FDA Warning On the Company – Cases and Tools in Biotechnology Management

Pharmas with up-and-coming JAK inhibitors face 'shrinking' potential after FDA crackdown | Fierce Biotech
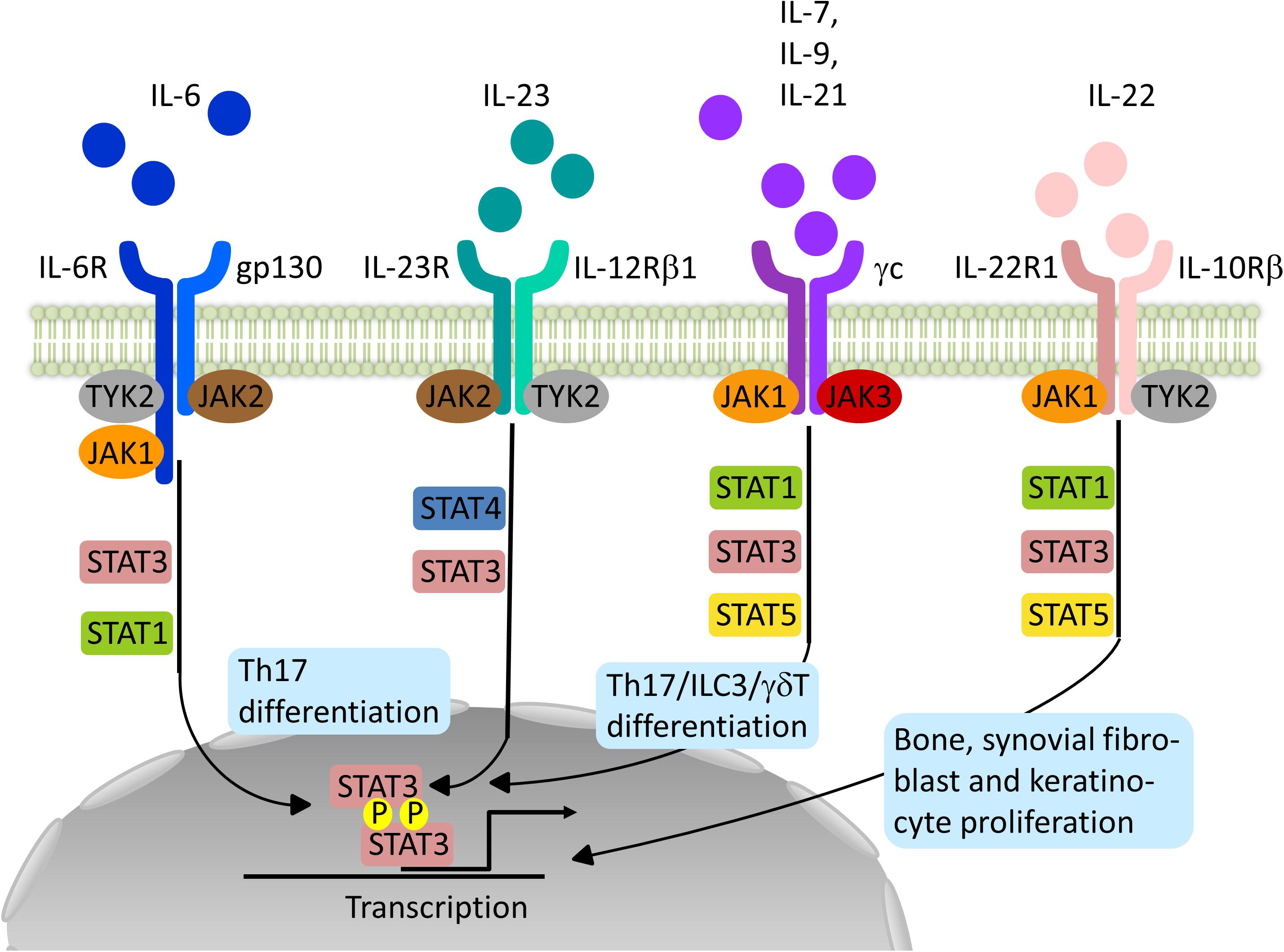
Frontiers | Impact of Janus Kinase Inhibition on the Treatment of Axial Spondyloarthropathies | Immunology

FDA approves Pfizer's JAK1 inhibitor Cibinqo for moderate-to-severe atopic dermatitis - Drug Discovery and Development
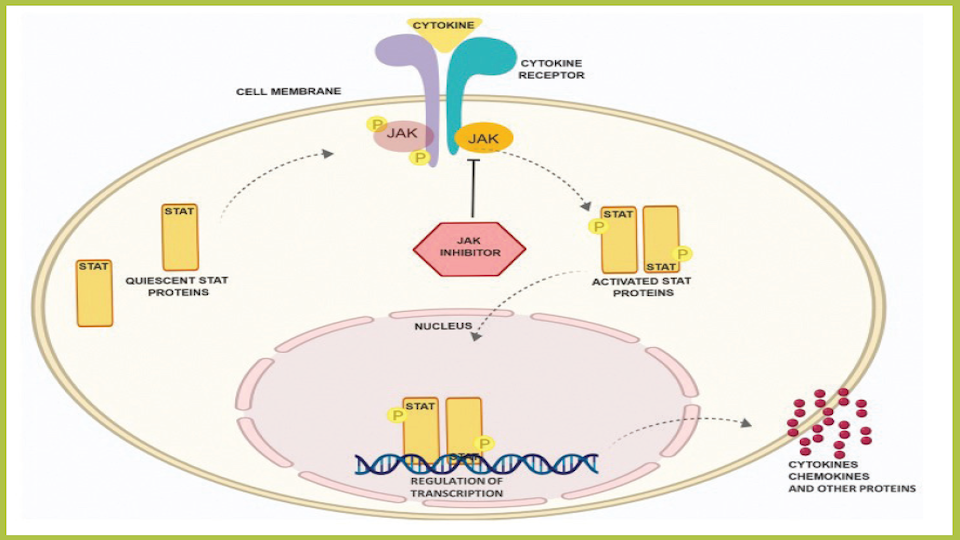
JAK Inhibitors Are Coming and They Are the Biggest Eczema Development in Years | National Eczema Association

Janus kinase inhibitors in dermatology: Part II. A comprehensive review - Journal of the American Academy of Dermatology




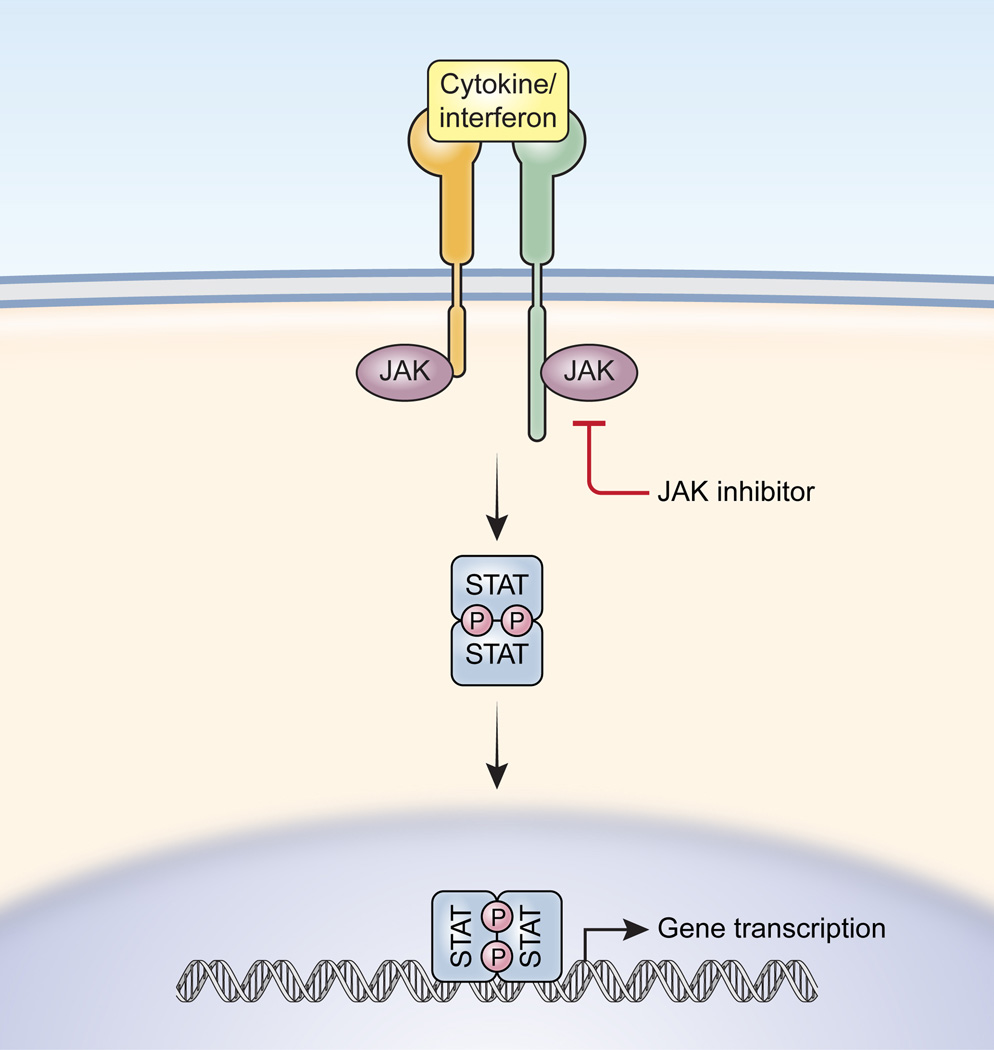
:max_bytes(150000):strip_icc()/jak-inhibitors-4706526-FINAL-3461d4fc6ce44f54801ef59489afa62c.png)